Organic Reaction Mechanisms (Curly Arrow)

Organic reactions are involved with a single basic mechanism that is a negative part of one reactant attacks on the positive site of other reactant and forms the final organic product. Most of the organic reactions are working on this single concept that negative part is attacking on positive part and some part getting substituted, […]
E1 vs. E2 elimination reactions

S. No. E1 elimination Reaction E2 elimination Reaction 1. Unimolecular (Rate of reaction depends on one molecule that is alkyl halide) Bimolecular (Rate of reaction depends on two molecules that is alkyl halide and base) 2. First order of reaction Second order of reaction 3. Two step reaction One step reaction 4. Involves weak base […]
Ozonolysis
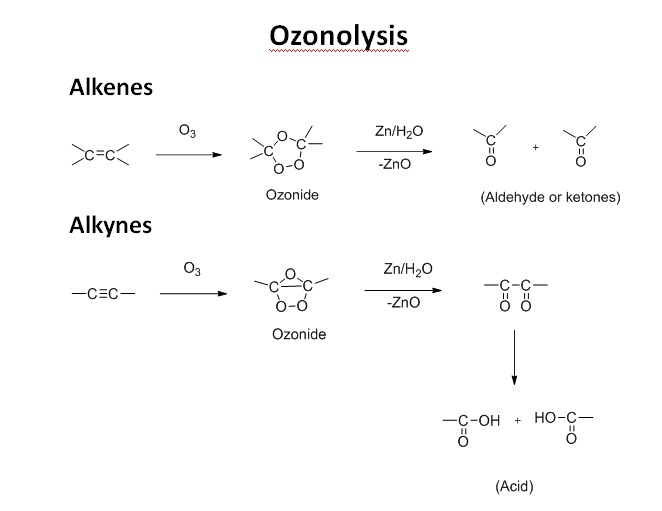
Ozonolysis of Alkenes and Alkynes Mechanism:
Geometric Isomerism
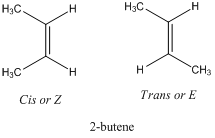
Alkenes show the geometric isomerism which is denoted by E/Z-isomers. These differ in the orientation of different atoms or substituents across the double bond. They are also referred as stereoisomer particularly diastereomers. For example, 2-butene has two geometric isomers. They also differ in physical properties. The prefix “Cis” or “Z” comes from Latin word which […]
Markovnikov’s Rule
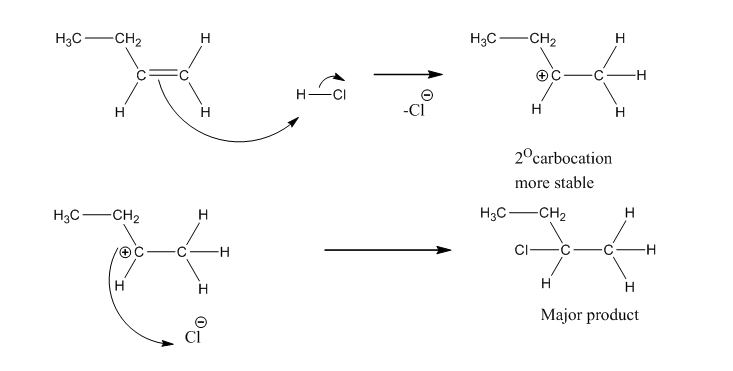
Valadimir Markovnikov observed the pattern of halogenation of alkene where formations of two isomeric products are possible. Markovnikov rule state that in the addition of acid to carbon-carbon double bond of alkene, the hydrogen of acid attaches to the carbon that already holds a greater number of hydrogen. It’s a regioselective reaction because the addition […]
Anti-Markovnikov Rule
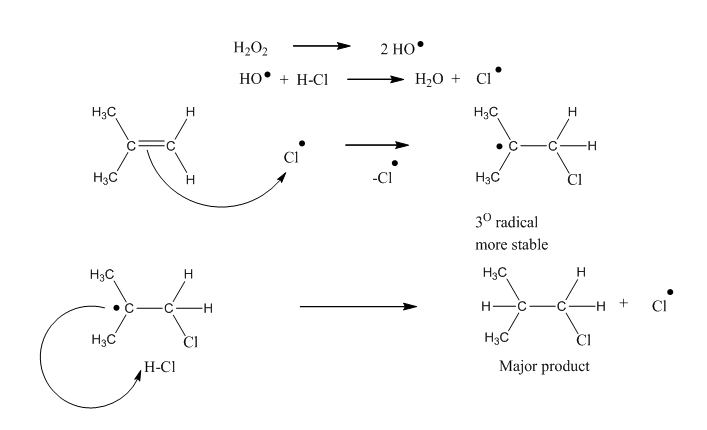
The addition of hydrogen halide to an alkene in the presence of peroxide is opposite to Markovnikov rule. This reversal of orientation of product is peroxide effect and also known as Kharasch effect observed by M.S. Kharasch and F.R. Mayo in 1973. So, in the presence of peroxide addition of hydrogen on carbon-carbon double bond […]
Boiling Point of an Organic Compound
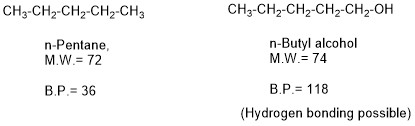
When we talk about boiling point of organic molecules, it is the temperature at which breakage of attraction force (like Vanderwaal attraction force) and bonds (like hydrogen bonds) between the molecules takes place and molecules get free to move separately, there is no breakage of covalent bond within a molecule at its boiling point. Boiling […]
Melting Point of an Organic Compound
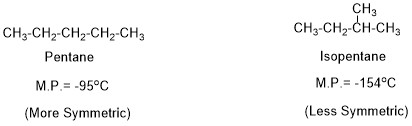
The most important application of melting point is confirmation of synthesized organic compound, however now different spectral techniques are also available (like IR, NMR and Mass Spectroscopy) but melting point confirmation through reference melting point is an economical, easy and convenient way for confirming the synthesized compound. Melting point (M.P.) means the temperature at which […]
Hydrogenation of Alkenes over Nickel Catalyst
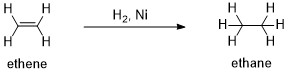
Alkenes (unsaturated hydrocarbon) get reduced to alkanes in the presence of hydrogen and nickel catalyst into alkanes (saturated hydrocarbon). In this process, nickel catalyst adsorbs the alkene and hydrogen molecules on its surface, and then hydrogen gets attached to alkenes making new sigma bonds and finally converting alkenes into alkanes.
Rearrangement Reactions in Organic Chemistry
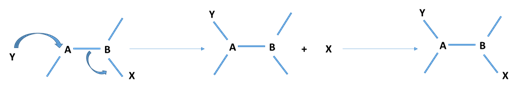
Rearrangement reactions are one of the important class of organic reactions, wherein a group or bond attached to a carbon atom moves to another carbon atom often in a single molecule. Example: A-B-X → B-A-X In rearrangement reactions, the reaction may involve more than one step. No by-products would be formed, and products would be […]