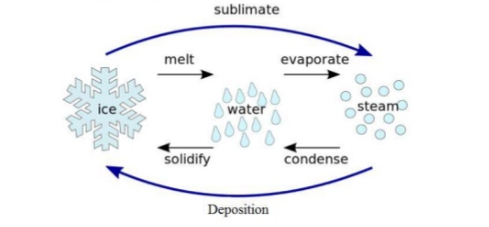
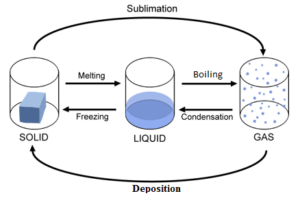
Sublimation
It is a process in which a solid substance is directly converted into the gas phase, skipping the liquid phase. It is an endothermic process in which pressure and temperature are below the triple point of substance. Reversing the sublimation process is known as desublimation. The term sublimation is used to give an idea about physical change, not a chemical reaction.
Sublimation takes place when the heat is completely absorbed which provides sufficient energy to leave the attractive forces of the nearby molecule and turn into the vapor phase. The required energy is called the enthalpy of sublimation.
 Application:
Application:
 Application:
Application:
- Printing and digital printing done using low sublimation but the dyes used remain the same.
- Image printing on any kind of fabric.
- The final image obtained are brilliant due to dye bonding.
- Screen-printing, embroidery, and appliques are made using dye sublimation.
- No need to use half-screen printing because it can produce tons that are equal to photographs.