Baker-Venkataraman Rearrangement
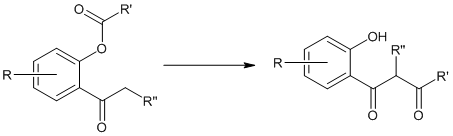
This rearrangement reaction of organic chemistry involves the regio-selective formation of 1,3-diketones through the base-induced transfer of acyl group in O-acylated phenol ester. It is an intramolecular acyl transfer reaction via the formation of an enolate. This rearrangement reaction plays a key role in the synthesis of flavons from readily available starting materials. Where R’ […]
Benzyl-Benzilic acid Rearrangement
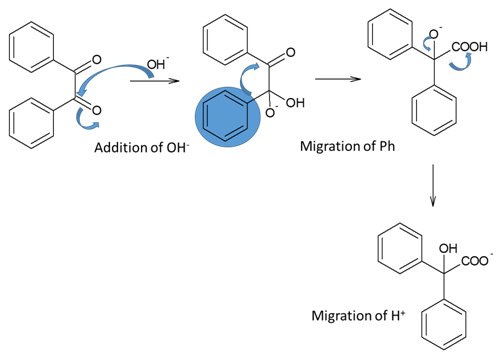
Rearrangement of a-diketones to a-hydroxycarboxylic acid through 1, 2-shift intramolecular rearrangement is called as a benzyl-benzylic acid reaction. This reaction helps in synthesizing a-hydroxy acids using easily available starting materials. Mechanism involving in this rearrangement reaction is,
Claisen Rearrangement
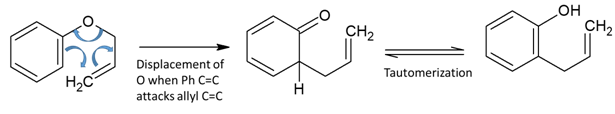
Sigmatropic or electrocyclic intramolecular rearrangement of aryl allyl ether in to gamma, delta-unsaturated carbonyl compound in the presence of heat or Lewis acid is known as Claisen rearrangement. Sigmatropic rearrangement involves migration of s bond from one end of p system to others. Reaction mechanism of this reaction closely resembles that of Diels- Alder reaction […]
Cope Rearrangement
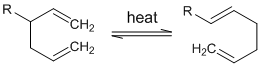
Thermal isomerization of 1,5-diene into regio-specific 1,5-diene is known as cope rearrangement. This rearrangement is generally reversible in nature and is key in the synthesis of complex natural products such as alkaloids, carbohydrates, etc. in organic chemistry. Mechanism of Cope and Claisen rearrangements are very similar. Anionic oxy-cope rearrangement is the reaction in […]
Fries Rearrangement
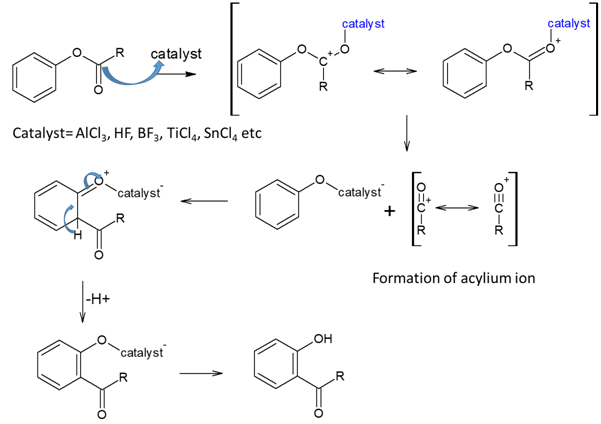
It is an organic reaction wherein acyl phenols are formed from phenolic esters in the presence of a Lewis acid catalyst. Many of these are act as intermediates in the pharmaceutical, agricultural, thermographic and other industrial synthetic products. When the same rearrangement reaction takes place in the presence of light, it is known as photo-fries […]
Pinacol Rearrangements
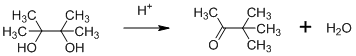
Acid-catalyzed conversion of 1,2-diols to ketones along with the elimination of water as a by-product are collectively called as pinacol rearrangements. One of such organic reaction is the formation of pinacolone (methyl tert-butyl ketone) from pinacol (2,3-dimethyl-2,3-butanediol) and is famously known as Pinacol-pinacolone rearrangement. This reaction used only occasionally to synthesize ketones which are very […]
Classification of Organic Compounds

Organic compounds are classified according to the type of elements and bonds present in the molecules. Compounds containing only carbon and hydrogen are called hydrocarbons. However organic compounds can also contain elements other than carbon and hydrogen-like oxygen, nitrogen, sulfur, phosphorus, silicon, etc. Hydrocarbons are further classified as aliphatic and aromatic hydrocarbons based on their […]
Methane
Methane is the smallest member of the alkane family as well as all of the organic compounds. Yet, It is the most important and powerful of gases on the planet. It is formed by the anaerobic decay of plants and is a major constituent of the natural gas. It can be obtained as a pure […]
Chemical Properties of Methane (Oxidation)

Methane reacts with reactive substances under vigorous conditions. One such reactive substance is oxygen. Oxidation of methane or in other words combustion (complete) of methane (in fact for any hydrocarbon) results in formation carbon dioxide and water with release of heat. This oxidation is the major reaction occurring during the burning of natural gas. Combustion […]
Halogenation of Methane
Methane reacts with most of the halogens except iodine. While it reacts vigorously with fluorine at room temperature and even at the dark condition, reactivity gradually goes down from chlorine to bromine. Methane interacts with chlorine at 250-400 °C or under the influence of ultraviolet rays (UV). The resulting products are a mixture of alkyl […]