Factors affecting basicity of Primary Amines
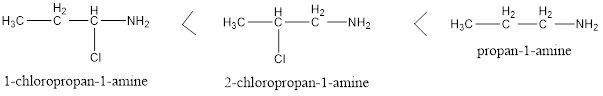
Factors Affecting Basicity of Primary Amines Two types of groups can be there in the structure of primary amines, one is electron withdrawing and other is electron donating. Electron donating groups increases the basicity and Electron withdrawing groups decreases the basicity of amines as their basicity depends on the availability of free lone pair present […]
Bartoli Indole Synthesis
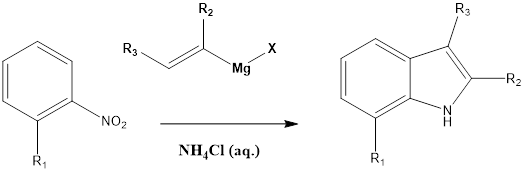
Bartoli Indole Synthesis This reaction involves the formation of substituted indoles from nitroarene in presence of an excess of vinyl Grignard reagent and aqueous ammonium chloride. The reaction was discovered by Giuseppe Bartoli and his team in the year 1989. The ortho-substituted nitroarene is preferred as they give a good yield. The bulkier group also […]
Corey-Kim Oxidation
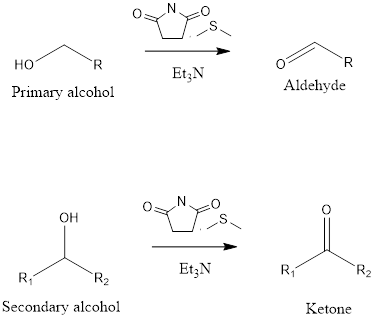
Corey-Kim Oxidation It is an organic reaction to form aldehydes and ketones from primary and secondary alcohol. The reaction takes place in presence of N-chlorosuccinimide(NCS), dimethylsulfide(DMS) and triethylamine(TEA). The oxidation reaction was discovered by Nobel laureate Eliar James Corey, American chemist and Choung Un Kim, Korean American chemist in the year 1972. In the mechanism […]
Ullmann Reaction
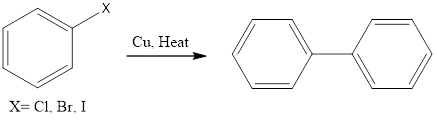
Ullmann Reaction Ullmann reaction is coupling organic chemistry reaction which forms biaryl by coupling of two molecules of aryl halide in the presence of copper metal and elevated temperature (200°C). The reaction was discovered by Fritz Ullmann. Mechanism of this organic reaction is not clear exactly. There are two proposed mechanisms first is the radical […]
Jones Oxidation
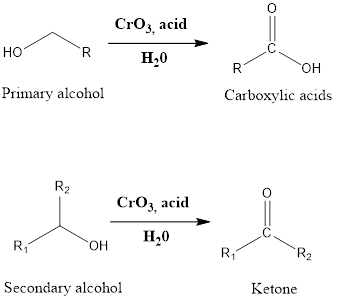
Jones Oxidation It is an oxidation reaction of organic chemistry which leads to the formation of carboxylic acid and ketone. The primary alcohol in presence of chromic trioxide, and sulfuric acid in a mixture of acetone-water (which is also referred as Jones reagent) forms carboxylic acid while secondary alcohol in presence of Jones reagent leads […]
Johnson-Claisen Rearrangement
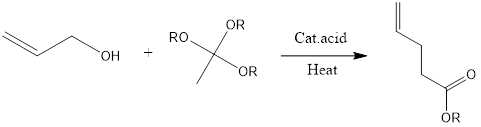
Johnson – Claisen Rearrangement Johnson-Claisen rearrangement is organic chemistry reaction to form unsaturated ester from allylic alcohol and trialkyl orthoacetate in presence of mildly acidic conditions and heat. The reaction was discovered in 1970 by W.S Johnson and its co-workers. Mechanism of reaction– Firstly alkoxide group is protonated of orthoacetate. Then molecule of alcohol is […]
Biginelli Reaction
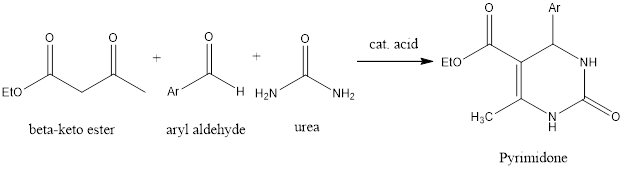
Biginelli Reaction Bignelli reaction is ring forming organic chemistry reaction which forms pyrimidones from three components aldehyde, β-keto ester, and urea in the presence of acidic conditions. The reaction was discovered by Italian chemist Pietro Biginelli in 1891. Mechanism of reaction – Firstly, in reaction condensation of aldehyde and urea takes place, as it happens […]
Eschenmoser-Claisen Rearrangement

Eschenmoser – Claisen Rearrangement Eschenmoser-Claisen rearrangement is the organic chemistry reaction which leads to the formation of α, β unsaturated amide from allylic alcohol in presence of heated N,N dimethylacetamide dimethyl acetal. The reaction is named after Albert Eschenmoser who discovered this reaction in 1964. This reaction is also used in synthesis of morphine (opioid […]
Finkelstein Reaction
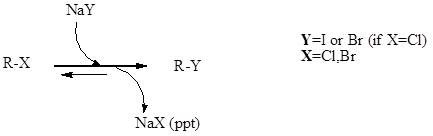
Finkelstein Reaction Finkelstein reaction is a single step substitution nucleophilic bimolecular reaction (SN2) which involves the halide replacement. The reaction was discovered by Hans Finkelstein a German chemist. It is a type of equilibrium process and it is carried forward as acetone has poor solubility for formed metal halide which involves Le Chatelier’s principle. The […]
Eschweiler- Clarke Reaction
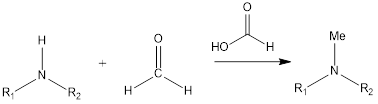
Eschweiler – Clarke Reaction Eschweiler- Clarke reaction is a substitution organic chemistry reaction which leads to the formation of tertiary methylamines by reaction of primary amine or secondary amine in presence of formaldehyde and formic acid. The reaction was discovered by German chemist Wilhelm Eschweiler as well as British chemist Hans Thacker Clarke. Mechanism of […]
