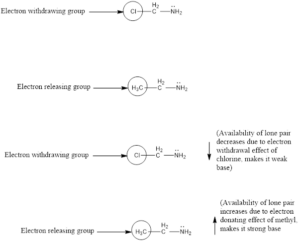
Factors Affecting Basicity of Primary Amines
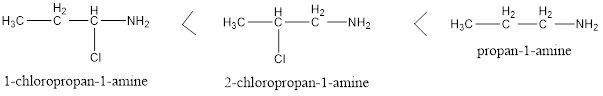
Two types of groups can be there in the structure of primary amines, one is electron withdrawing and other is electron donating. Electron donating groups increases the basicity and Electron withdrawing groups decreases the basicity of amines as their basicity depends on the availability of free lone pair present on nitrogen, electron donating group increases the density of electrons on nitrogen to easily accept proton thereby increased basicity and vice versa. Also, nearness of these groups to amines creates more impact on basicity compared to the groups away from amine group whether it is withdrawing or donating. For example, the following is a basicity order: