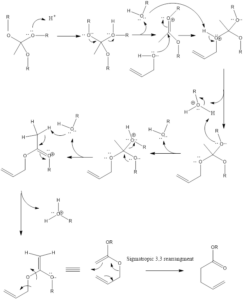
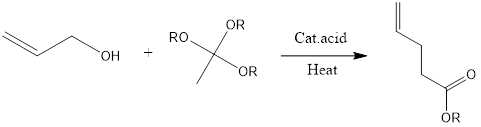
Johnson - Claisen Rearrangement
Johnson-Claisen rearrangement is organic chemistry reaction to form unsaturated ester from allylic alcohol and trialkyl orthoacetate in presence of mildly acidic conditions and heat. The reaction was discovered in 1970 by W.S Johnson and its co-workers.
Mechanism of reaction– Firstly alkoxide group is protonated of orthoacetate. Then molecule of alcohol is released from protonated alkoxide. There is the formation of oxonium cation which is further attached upon by alcohol. Then another alkoxide gets protonated, again alcohol molecule is released and there is the formation of 1,5-diene intermediate that goes through sigmatropic rearrangement leading to the formation of unsaturated ester product.