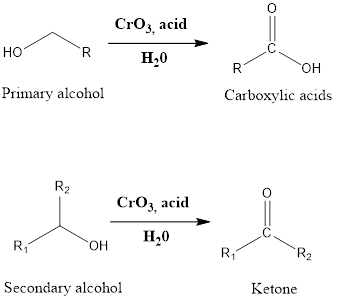
Jones Oxidation
It is an oxidation reaction of organic chemistry which leads to the formation of carboxylic acid and ketone. The primary alcohol in presence of chromic trioxide, and sulfuric acid in a mixture of acetone-water (which is also referred as Jones reagent) forms carboxylic acid while secondary alcohol in presence of Jones reagent leads to the formation of the ketone. The reaction was first discovered by Ewart Jones and that’s why the reaction is named as Jones oxidation.
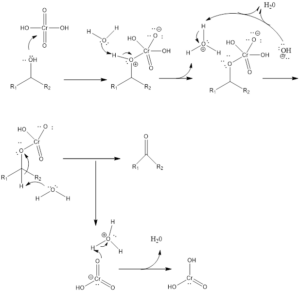
Mechanism of Organic Reaction– Firstly there is the formation of chromic acid by reaction of chromic trioxide with acid. Chromic acid undergoes oxidation of alcohol which forms chromate ester as an intermediate and by the intermolecular or intramolecular reaction in presence of water forms carbonyl compound. Aldehyde formed in presence of water gets hydrated and then oxidized leading to the formation of carboxylic acids.
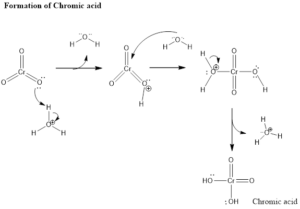
Formation of Ketone
The reaction has application in the formation of salicylic acid which is a precursor of aspirin, Methcathinone a psychoactive stimulant is the oxidation product of alcohols in presence of Jones reagent and this reaction also helps in the formation of aminoindans.