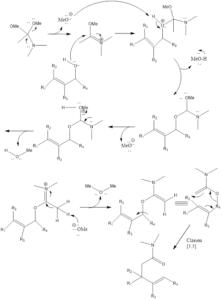
Eschenmoser - Claisen Rearrangement
Eschenmoser-Claisen rearrangement is the organic chemistry reaction which leads to the formation of α, β unsaturated amide from allylic alcohol in presence of heated N,N dimethylacetamide dimethyl acetal. The reaction is named after Albert Eschenmoser who discovered this reaction in 1964. This reaction is also used in synthesis of morphine (opioid analgesic).
Mechanism of organic reaction– Firstly in reaction methoxide group leaves from amide which leads to formation of iminium cation, that gets further attacked by alcohol. Then proton transfer occurs, on methoxide group that gets released in form of methanol molecule leading to formation of again iminium cation. Methoxide causes deprotonation to form 1,5-diene intermediate. Intermediate formed gone through Claisen sigmatropic rearrangement to form final amide product.