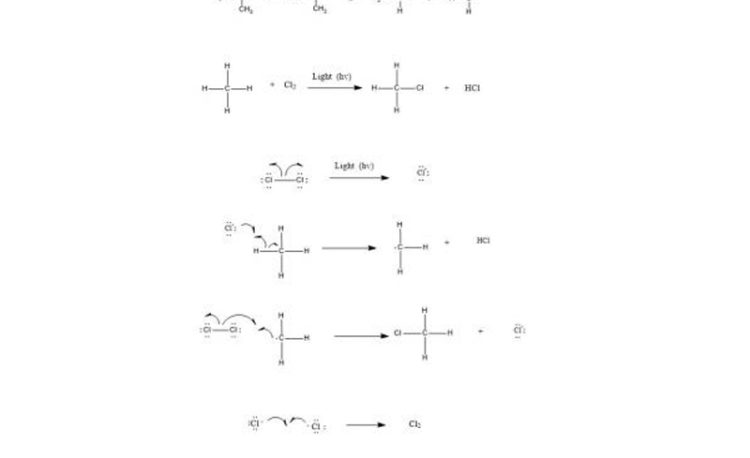
Free Radicals Halogenation of Alkane
Free radicals are highly reactive species with unpaired valance electron. Carbon free radicals are formed during halogenation of alkanes and orientation as well as reactivity depends on the type of free radical. It is known as free radical substitution reaction.
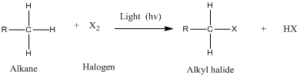 Ease of formation of free radical: 3° > 2° > 1° > CH3. It is because of more stable radical forms easily.
Ease of formation of free radical: 3° > 2° > 1° > CH3. It is because of more stable radical forms easily.
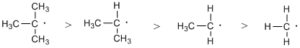 Free radicals halogenation is a chain reaction involving three distinct steps i.e. initiation, propagation, and termination.
Free radicals halogenation is a chain reaction involving three distinct steps i.e. initiation, propagation, and termination.
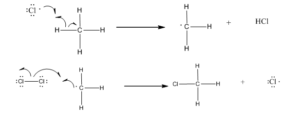
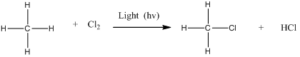 Step 1: Initiation reactions involve the formation of free radicals.
The formation of radicals is through breaking of covalent bonds by hemolysis.
Step 1: Initiation reactions involve the formation of free radicals.
The formation of radicals is through breaking of covalent bonds by hemolysis.
 Step 2: Chain propagation: Halogen free radical reacts with an alkane to form alkyl radical which reacts with halogen and produce alkyl halide and free radical.
Step 2: Chain propagation: Halogen free radical reacts with an alkane to form alkyl radical which reacts with halogen and produce alkyl halide and free radical.
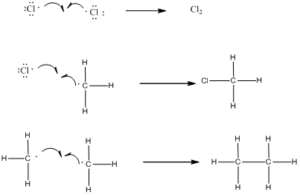
 Step 3: Chain Termination: Termination reactions involve the combination of two free radicals to form a more stable species.
Step 3: Chain Termination: Termination reactions involve the combination of two free radicals to form a more stable species.
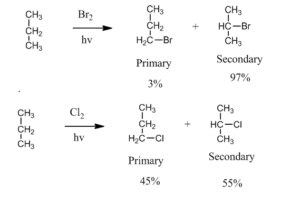
 Selectivity: If there is a possibility to form different types of an alkyl radical then depending on stability the tertiary is more prominent then secondary and so on.
Bromine is more selective than chlorine due to its less reactivity. For example bromination of propane is more selective than chlorination by a factor of 97 to 1 for secondary and primary halide respectively.
Selectivity: If there is a possibility to form different types of an alkyl radical then depending on stability the tertiary is more prominent then secondary and so on.
Bromine is more selective than chlorine due to its less reactivity. For example bromination of propane is more selective than chlorination by a factor of 97 to 1 for secondary and primary halide respectively.
 Ease of formation of free radical: 3° > 2° > 1° > CH3. It is because of more stable radical forms easily.
Ease of formation of free radical: 3° > 2° > 1° > CH3. It is because of more stable radical forms easily.
 Step 1: Initiation reactions involve the formation of free radicals.
The formation of radicals is through breaking of covalent bonds by hemolysis.
Step 1: Initiation reactions involve the formation of free radicals.
The formation of radicals is through breaking of covalent bonds by hemolysis.
 Step 2: Chain propagation: Halogen free radical reacts with an alkane to form alkyl radical which reacts with halogen and produce alkyl halide and free radical.
Step 2: Chain propagation: Halogen free radical reacts with an alkane to form alkyl radical which reacts with halogen and produce alkyl halide and free radical.
 Step 3: Chain Termination: Termination reactions involve the combination of two free radicals to form a more stable species.
Step 3: Chain Termination: Termination reactions involve the combination of two free radicals to form a more stable species.
 Selectivity: If there is a possibility to form different types of an alkyl radical then depending on stability the tertiary is more prominent then secondary and so on.
Bromine is more selective than chlorine due to its less reactivity. For example bromination of propane is more selective than chlorination by a factor of 97 to 1 for secondary and primary halide respectively.
Selectivity: If there is a possibility to form different types of an alkyl radical then depending on stability the tertiary is more prominent then secondary and so on.
Bromine is more selective than chlorine due to its less reactivity. For example bromination of propane is more selective than chlorination by a factor of 97 to 1 for secondary and primary halide respectively.