Stability Order of Carbocation, Carbanion and Free Radicals

Stability order of carbocations increases as we move from primary to tertiary cation due to +I effect of methyl groups there is a redistribution of positive charge all over the molecule which reduces the intensity of positive charge on central carbon and increases the stability of the molecule. Stability order of carbanions decreases as we […]
Hydrocarbons
Most of the organic compounds are known as hydrocarbons because they are basically made up of hydrogen and carbon atoms. On the basis of their structure and arrangement, they are classified into two main classes, aliphatic and aromatic compounds. Aliphatic compounds can be classified further into families like alkanes, alkenes, alkynes, cyclic aliphatic compounds.
Specific Rotation
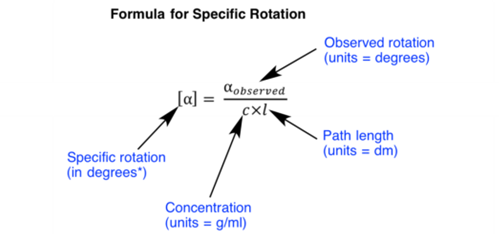
Specific rotation is characteristic value for every optically active compound. Observed rotation of a compound may vary as per variation in concentration and length of the sample tube, but the specific rotation is a constant value for every optically active compound. Following is a formula used for calculation of specific rotation: Practice Question: The concentration […]
Wedge and Dash Projection

In organic chemistry wedge and dash, projections are used to represent three-dimensional structures of compounds on two-dimensional papers. There are three types of bonds in the wedge and dash notations as shown in figure: Solid Lines: These lines show the groups or atoms connected in the plane of the paper. Wedges: These are wedge-shaped bonds […]
Resolution

The separation of a racemic mixture or racemic modification into pure enantiomers is called resolution. Resolution is a unique technique of separation because it is used to separate the compounds which are having same chemical and physical properties. The process of resolution involves the reaction of racemic acid or base with optically active reagent (base […]
Geometric Isomerism
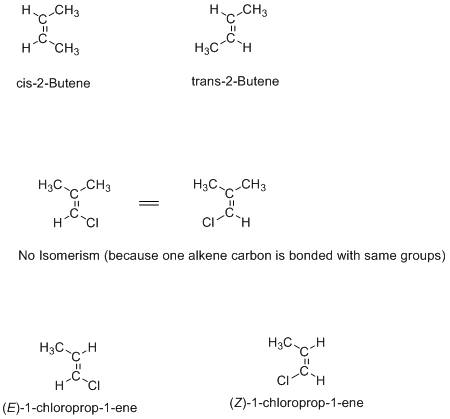
Geometric isomerism is shown by alkenes and cyclic compounds where rotation around the bond is not possible. These are normally defined as cis/trans or E/Z isomers. Examples are given below: Cis/trans terminology is used when the same type of substituents are attached to both double-bonded carbons, but when different groups attached to the both double-bonded […]
Electrophilic Substitution in Pyrrole (Reactivity and Orientation)
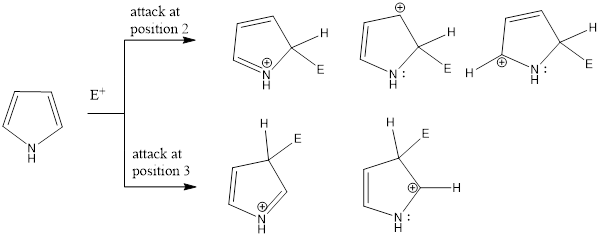
Pyrrole, thiophene, and furan gives electrophilic aromatic substitution reaction. These compounds are more reactive compared to benzene. Electrophiles majorly attack on 2nd position rather than 3rd position in these heterocyclic compounds. The reason behind it is the more number of resonating intermediate structure are possible to accommodate the positive charge when electrophile attacks on 2nd […]
Nucleophilic Aromatic Substitution (SNAr)
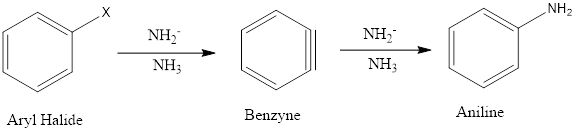
Ordinarily aromatic benzene ring and their derivatives give electrophilic aromatic substitution due to the high density of electrons over the ring but nucleophilic aromatic substitution can be seen in some specific conditions like aryl ring must contain strongly electron withdrawing groups on ortho and/or para positions to facilitate the nucleophile attack on the aromatic ring. […]
Electrophile Attack Orientation in Disubstituted Benzenes
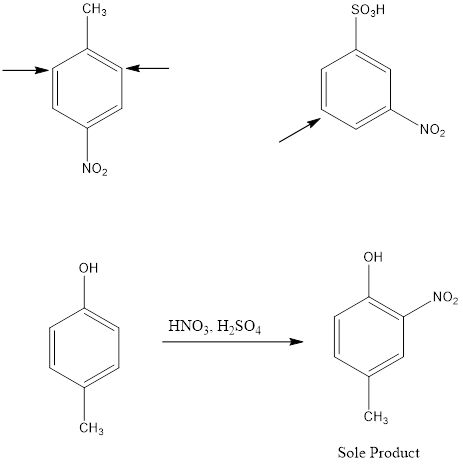
Disubstituted benzenes have two substituents on the ring which makes the orientation of incoming electrophile more complicated. There are two possibilities; one is when the two substituents are located in such a way that one reinforces the other and on the other hand the directive effect of one group opposes that of the other. In […]
Electrophilic addition to conjugated Diene
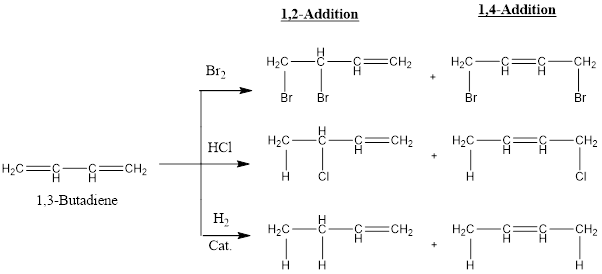
When we react bromine or HCl or catalytic hydrogen with 1,3-butadiene not only expected 1,2-addition product obtained but 1,4-addition product is also obtained which confirms the resonance of conjugated diene system. So following resonance hybrids are possible in conjugated diene system: