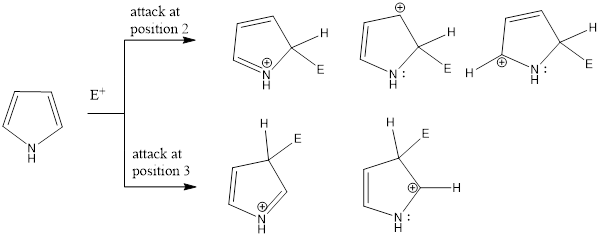
Electrophilic Substitution in Pyrrole (Reactivity and Orientation)
Pyrrole, thiophene, and furan gives electrophilic aromatic substitution reaction. These compounds are more reactive compared to benzene. Electrophiles majorly attack on 2nd position rather than 3rd position in these heterocyclic compounds. The reason behind it is the more number of resonating intermediate structure are possible to accommodate the positive charge when electrophile attacks on 2nd position (three resonating intermediate structures) that makes the intermediate carbocation more stable while if electrophile attacks on the 3rd position then only two resonating intermediate structures are possible as shown in the figure which is comparitively less stable. That’s why the attack of electrophiles takes place at 2nd position in pyrrole, thiophene, and furan.