Wolf - Kishner Reduction and Huang Minlon Modification
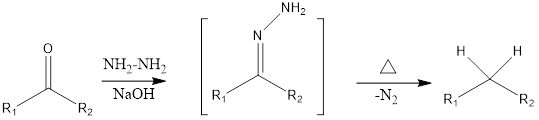
Wolf-Kishner reduction is the reaction, where carbonyl compounds aldehydes and ketones convert to corresponding alkanes. The reaction takes place in presence of hydrazine, base, and heat.
In this reaction, intermediate hydrazone is formed by condensation of the carbonyl substrate with hydrazine.
This reduction reaction was discovered by Ludwif Wolff and Nikolai Kishner in an early 19th century. N.Kishner in 1911, did reaction on already formed hydrazone in presence of hot potassium hydroxide which consisted of the crushed platinized porous plate. Wolf in 1912 heated ethanolic solution of hydrazones to 180 °C in the sealed tube along with sodium ethoxide.
Mechanism of action: Firstly the carbonyl substrate gets converted to hydrazone derivative, by a nucleophillic attack of hydrazine with a carbonyl group. This step is a slow step and referred to as the rate determining step. Then nitrogen of hydrazone is deprotonated in presence of a base and deprotonated hydrazone undergoes protonation. Then nitrogen is deprotonated and this rearrangement forms carbanion and nitrogen gas is released. The carbanion gets a proton from water which finally forms the corresponding alkane product.
Huang Minlon Modification
It is the modified Wolf-Kishner reduction reported by Huang Minlon in the year 1946, to overcome the limitations of the original reaction.
The modification involves refluxing the carbonyl substrate with 85% hydrazine hydrate and sodium hydroxide in ethylene glycol. Then further distillation is done to remove excess hydrazine and water from hydrazone, followed by raising the temperature to 200°C to remove nitrogen gas.
Ethylene glycol is used as it has high boiling point removes nitrogen gas which is produced as a side product in the reaction.
The modification provides advantages like the production of pure products with increased yield than the original reaction and it can be also used for sterically hindered ketones.