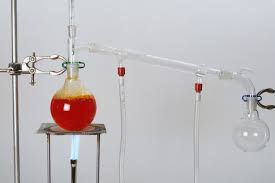
Distillation
Distillation is used to separate the pure chemical out of the liquid mixture. First evaporation takes place than condensation. The basic principle of distillation is the use of temperature based upon the temperature difference between the liquids gets separated.
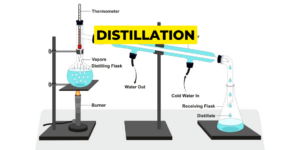 There many types of distillation:
There many types of distillation:
 There many types of distillation:
There many types of distillation:
- Simple Distillation
- Flash Distillation
- Vacuum Distillation
- Steam Distillation
- Molecular Distillation
- Fractional Distillation
- Azeotropic Distillation
- Destructive Distillation
- It is helpful in separating volatile oils such as clove distillation.
- Organic solvents can be purified based upon this distillation process example is absolute alcohol.
- To make official preparations like the spirit of ammonia, ether.
- It is also used in quality control methods like alcohol content in the elixir.
- In the refining industry of petroleum.
- Recovery of costly solvents.
- It can not be used for those liquids having comparable volatility.
- Flash distillation is not efficient in providing pure components.
- It is important to know which compound is applied to which distillation or else nothing will be obtained.