Stability Order of Carbocation , Carbanion and Free Radicals
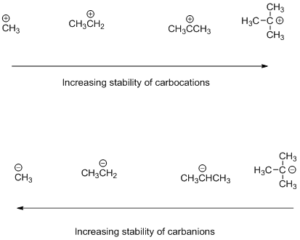
 Stability order of carbocations increases as we move from primary to tertiary cation due to +I effect of methyl groups there is a redistribution of positive charge all over the molecule which reduces the intensity of positive charge on central carbon and increases the stability of the molecule.
Stability order of carbanions decreases as we move from primary to tertiary anion because due to +I effect of methyl groups there is an increased intensity of negative charge on central carbon of tertiary carbanion which further makes it unstable.
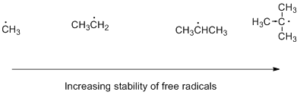
Stability order of free radicals increases as we move from primary to tertiary free radicals, due to +I effect of methyl groups there is a redistribution of lack of electron all over the molecule which reduces the intensity of lack of electron on central carbon and increases the stability of the molecule.
Stability order of carbocations increases as we move from primary to tertiary cation due to +I effect of methyl groups there is a redistribution of positive charge all over the molecule which reduces the intensity of positive charge on central carbon and increases the stability of the molecule.
Stability order of carbanions decreases as we move from primary to tertiary anion because due to +I effect of methyl groups there is an increased intensity of negative charge on central carbon of tertiary carbanion which further makes it unstable.
Stability order of free radicals increases as we move from primary to tertiary free radicals, due to +I effect of methyl groups there is a redistribution of lack of electron all over the molecule which reduces the intensity of lack of electron on central carbon and increases the stability of the molecule. 


