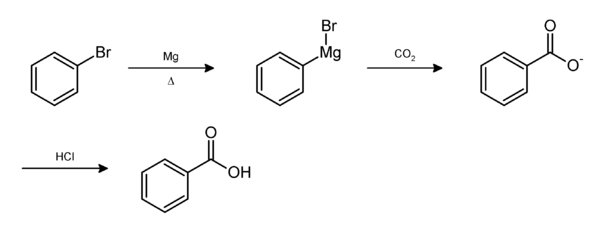
Solubility of Benzoic Acid Versus Phenol in Aqueous Solution of NaOH and NaHCO3
Benzoic acid and phenol both are insoluble in water due to non-polar benzene ring but these molecules become soluble if we react them with an aqueous solution of NaOH, which forms water-soluble sodium salt of the benzoic acid (Sodium benzoate) and the sodium salt of phenol (sodium phenoxide). But when these compounds reacted with an aqueous solution of NaHCO3, benzoic acid reacted, but phenol is unable to react as it is comparatively weaker acid, so unable to form a salt with a weaker base (NaHCO3).