Atomic Orbital vs. Molecular Orbital
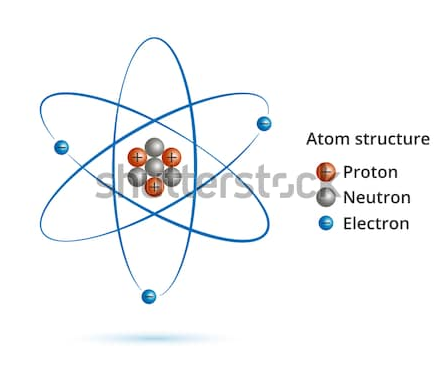
Atoms are building block and smallest part of the element which reacts chemically. Atoms consist of three fundamental particles i.e. electrons, protons, and neutrons. Charged particles, electrons they move around the nucleus in well-defined circular orbit. Atoms in the group are called as a molecule, a chemical bond is present which holds atoms in the molecule.
Orbitals they represent different energy levels. An electron in orbital has a definite energy. Orbital is a mathematical function describing wave properties of matter which helps in calculating the probability of an electron in a specific place around the nucleus of an atom.
Atomic orbital it is placed outside the nucleus in an atom, where electron finding probability is highest i.e. 95%. They are formed by electron cloud surrounding the atom. Around the nucleus, there are energy levels consisting group of atomic orbitals. The orbitals s, p, d, f they have characteristic energy related with it. There can be a maximum of 2 electrons and each orbital has a different shape and spatial orientation. Most of the organic molecules have their electrons in s and p orbitals. S orbital has a spherical shape like a hollow ball and it has its center at the nucleus of the atom. P orbital has dumb-bell shape and it has 3 orbitals px, py, pz. Each one contains two lobes and nucleus lies between them. There is a node at the nucleus where the probability of finding an electron is zero. Inorganic compounds present down the periodic table consists of d and f orbitals. The shape of d orbital like 2 dumb-bells in a plane and it consists of 2 nodes. There are five d orbitals dxy, dyz, dxz, dx2y2, and dz2 . There are 7 f orbitals which have a complicated shape.
Molecular orbital they are formed by the fusion of atomic orbitals having the same energy and it is a place where electron finding probability is highest in a molecule. The molecular orbital theory was developed in 1932 by F. Hund and R.S Mulliken. Characteristic features of the theory are: –
- The electrons which are present in molecule they are placed in various molecular orbitals similarly as electrons are placed in atomic orbitals.
- Atomic orbitals they should have same or nearly same energy, same symmetry about the molecular axis and maximum overlapping to form molecular orbital.
- The atomic orbital is monocentric and molecular orbital is polycentric because atomic orbital is influenced by one nucleus and molecular orbital by two or more nucleus.
- Molecular orbitals formed depend upon the number of atomic orbitals. If 2 atomic orbitals are combining they form 2 molecular orbitals. One is bonding and other is anti-bonding molecular orbital.
- The bonding molecular orbital has less energy than anti-bonding molecular orbital, therefore bonding molecular orbital has greater stability.
The atomic orbitals they are expressed by Schrodinger wave equation as wave function while molecular orbitals are expressed by linear combination of atomic orbitals. There are subtraction and addition of atomic orbitals due to constructive and destructive interference leads to the formation of antibody orbital which has higher energy than that of atomic orbitals and bonding orbital which has lower energy than that of atomic orbitals.
There is sigma molecular orbital which is formed always by the direct or head on the overlap of 1s, two px, 1s and px atomic orbitals. Pi molecular orbital is formed by sideways overlapping of atomic orbitals like 2 pz, 2 py, py or pz, and dxz atomic orbital.