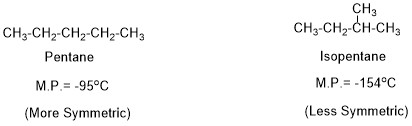Melting Point of an Organic Compound
The most important application of melting point is confirmation of synthesized organic compound, however now different spectral techniques are also available (like IR, NMR and Mass Spectroscopy) but melting point confirmation through reference melting point is an economical, easy and convenient way for confirming the synthesized compound.
Melting point (M.P.) means the temperature at which a solid melts into liquid form. M.P. is affected by various factors; one of them is an intermolecular force between the molecules. Stronger the force (like ionic force, e.g., Na+Cl–) higher the melting point, weaker the force (like Vander Wall, dipole-dipole, hydrogen bonding, e.g., Butane, butyric acid, etc.) between molecules, lower the melting point.
 Another important factor affecting melting point is a symmetry of the molecules. More symmetric the molecule, it can form more compact solid crystalline form. Thus its M.P. would be higher compared to a less symmetric compound which can not fit with each other more compactly.
Another important factor affecting melting point is a symmetry of the molecules. More symmetric the molecule, it can form more compact solid crystalline form. Thus its M.P. would be higher compared to a less symmetric compound which can not fit with each other more compactly.
 Another important factor affecting melting point is a symmetry of the molecules. More symmetric the molecule, it can form more compact solid crystalline form. Thus its M.P. would be higher compared to a less symmetric compound which can not fit with each other more compactly.
Another important factor affecting melting point is a symmetry of the molecules. More symmetric the molecule, it can form more compact solid crystalline form. Thus its M.P. would be higher compared to a less symmetric compound which can not fit with each other more compactly.