Hybridization
Hybridization is defined as the mixture of two different molecular orbitals to form a new orbital/hybrid having different energy and shape.
Types:
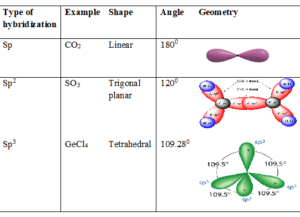
Sp, Sp2, Sp3, Sp3d, Sp3d2, Sp3d3.
Sp hybridization:
It is done by intermixing one ‘s’ and one ‘p’ orbital. Finally, originated hybrid has an energy of two different orbitals and hybrid is called a Sp hybrid and the process is known as hybridization.
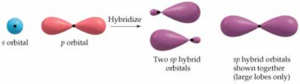
These are the orbitals arranged at an angle of 1800. Containing half portion of s and half portion of p. An example of Sp hybridization is Acetylene.
Sp2 hybridization:
The formation of sp2 hybridization occurs when carbon has 3 groups are attached to it. It involves 67% of p orbital and 33% of s orbital to produce a hybrid.
These are having a shape like a triangle as three corners are at an angle of 1200. It requires a two-sigma bond and one pi bond. An example is an ethylene.
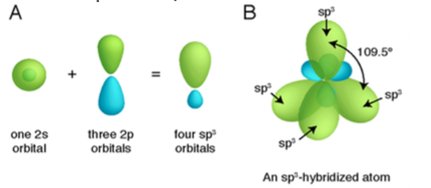
Sp3 hybridization:
It occurs when carbon has four groups attached with it where s character is only 25% and p contains almost 75% of the portion of a hybrid.
It has a tetrahedral shape which is found at an angle of 109.280. One such example is methane.
Summary: