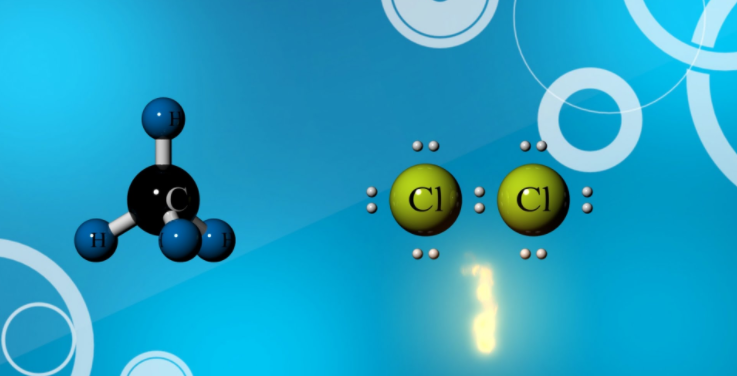
Halogenation of Methane
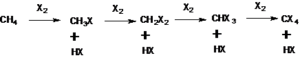
Methane reacts with most of the halogens except iodine. While it reacts vigorously with fluorine at room temperature and even at the dark condition, reactivity gradually goes down from chlorine to bromine. Methane interacts with chlorine at 250-400 °C or under the influence of ultraviolet rays (UV). The resulting products are a mixture of alkyl halides. Thus reaction of methane with chlorine in the presence of UV light will produce methyl chloride, dichloromethane, trichloromethane (chloroform), and carbon tetrachloride.
 Reactivity of halogens X2 -> F2 > Cl2 > Br2 > I2 (almost unreactive)
The reaction follows a free radical mechanism and is initiated by dissociation of chlorine to chlorine radical under the influence of UV light or heat. The chlorine radical reacts with methane to generate methyl radical which later reacts with chlorine or combine chlorine radical to form methyl chloride. The same sequence of reaction (chain reaction) is followed by the formation of other products starting from methyl chloride as a reactant.
Reactivity of halogens X2 -> F2 > Cl2 > Br2 > I2 (almost unreactive)
The reaction follows a free radical mechanism and is initiated by dissociation of chlorine to chlorine radical under the influence of UV light or heat. The chlorine radical reacts with methane to generate methyl radical which later reacts with chlorine or combine chlorine radical to form methyl chloride. The same sequence of reaction (chain reaction) is followed by the formation of other products starting from methyl chloride as a reactant.
 Reactivity of halogens X2 -> F2 > Cl2 > Br2 > I2 (almost unreactive)
The reaction follows a free radical mechanism and is initiated by dissociation of chlorine to chlorine radical under the influence of UV light or heat. The chlorine radical reacts with methane to generate methyl radical which later reacts with chlorine or combine chlorine radical to form methyl chloride. The same sequence of reaction (chain reaction) is followed by the formation of other products starting from methyl chloride as a reactant.
Reactivity of halogens X2 -> F2 > Cl2 > Br2 > I2 (almost unreactive)
The reaction follows a free radical mechanism and is initiated by dissociation of chlorine to chlorine radical under the influence of UV light or heat. The chlorine radical reacts with methane to generate methyl radical which later reacts with chlorine or combine chlorine radical to form methyl chloride. The same sequence of reaction (chain reaction) is followed by the formation of other products starting from methyl chloride as a reactant.