Geometric Isomerism
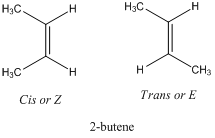
Alkenes show the geometric isomerism which is denoted by E/Z-isomers. These differ in the orientation of different atoms or substituents across the double bond. They are also referred as stereoisomer particularly diastereomers. For example, 2-butene has two geometric isomers. They also differ in physical properties.
The prefix “Cis” or “Z” comes from Latin word which means “on this side” whereas “Trans” or “E” means “across”.
So cis or Z -2-butene has methyl substituents on the same side whereas trans or E -2-butene has methyl substituents on opposite side.
Priority defined as per sequence rule and based on the high atomic number so if atoms with high priority are on the same side called as cis or Z whereas if atoms with high priority are on opposite side called as trans or E geometrical isomers.