Claisen Rearrangement
Sigmatropic or electrocyclic intramolecular rearrangement of aryl allyl ether in to gamma, delta-unsaturated carbonyl compound in the presence of heat or Lewis acid is known as Claisen rearrangement. Sigmatropic rearrangement involves migration of s bond from one end of p system to others.
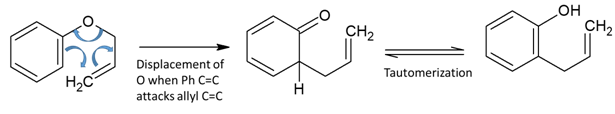
 Reaction mechanism of this reaction closely resembles that of Diels- Alder reaction and is:
Aliphatic Claisen rearrangement is similar to aromatic Claisen rearrangement allyl vinyl ether in to gamma, delta-unsaturated carbonyl compound.
Reaction mechanism of this reaction closely resembles that of Diels- Alder reaction and is:
Aliphatic Claisen rearrangement is similar to aromatic Claisen rearrangement allyl vinyl ether in to gamma, delta-unsaturated carbonyl compound.
 Reaction mechanism of this reaction closely resembles that of Diels- Alder reaction and is:
Aliphatic Claisen rearrangement is similar to aromatic Claisen rearrangement allyl vinyl ether in to gamma, delta-unsaturated carbonyl compound.
Reaction mechanism of this reaction closely resembles that of Diels- Alder reaction and is:
Aliphatic Claisen rearrangement is similar to aromatic Claisen rearrangement allyl vinyl ether in to gamma, delta-unsaturated carbonyl compound.