Boiling Point of an Organic Compound
When we talk about boiling point of organic molecules, it is the temperature at which breakage of attraction force (like Vanderwaal attraction force) and bonds (like hydrogen bonds) between the molecules takes place and molecules get free to move separately, there is no breakage of covalent bond within a molecule at its boiling point.
Boiling Point (B.P.) of any organic compound depends on its molecular weight, if molecular weight increases, B.P. also increases. Generally, 20-30oC B.P. increases by increasing the chain length by one carbon. If two compounds with same molecular weight then there are different factors which determine the boiling point of the organic compounds. Following are the possible factors:
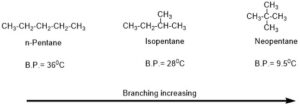
First, most important factor is the shape of the molecule, whether the molecule is branched or unbranched. If the molecule is more branched means had a spherical shape which causes lesser surface area and weaker Vanderwaal attraction force between the molecules thus, it would have a lesser boiling point. If organic compound is unbranched or less branched will have more surface area which causes more Vanderwaal attraction force between the molecules thus it would have a higher boiling point.
Example:
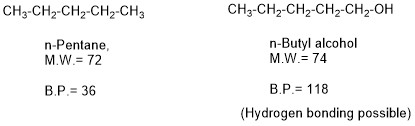
 Another important factor is a functional group of the organic molecule; if the functional group have polarity and have a capacity of making hydrogen bonds between the molecules, then the high temperature would be needed to break these hydrogen bonds along with Vanderwaal attraction force between the molecules thus B. P. would be higher of such type of compounds.
Example:
Another important factor is a functional group of the organic molecule; if the functional group have polarity and have a capacity of making hydrogen bonds between the molecules, then the high temperature would be needed to break these hydrogen bonds along with Vanderwaal attraction force between the molecules thus B. P. would be higher of such type of compounds.
Example:
 Another important factor is a functional group of the organic molecule; if the functional group have polarity and have a capacity of making hydrogen bonds between the molecules, then the high temperature would be needed to break these hydrogen bonds along with Vanderwaal attraction force between the molecules thus B. P. would be higher of such type of compounds.
Example:
Another important factor is a functional group of the organic molecule; if the functional group have polarity and have a capacity of making hydrogen bonds between the molecules, then the high temperature would be needed to break these hydrogen bonds along with Vanderwaal attraction force between the molecules thus B. P. would be higher of such type of compounds.
Example: