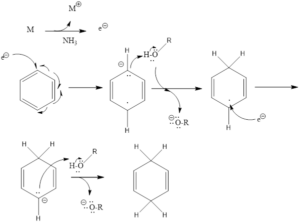
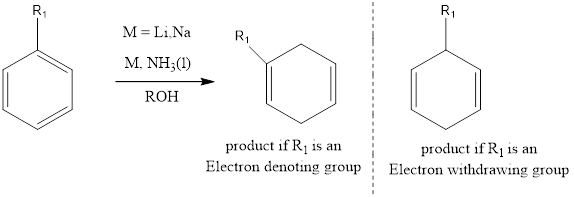
Birch Reduction
It is an organic chemistry reaction where the benzene ring containing aromatic compounds undergoes 1,4 reduction to produce unconjugated 1,4 cyclohexadienes. The reaction was discovered by Australian chemist Arthur Birch in the year 1944. The reaction takes place in presence of metals like lithium, sodium or potassium in liquid ammonium along with alcohol.
Mechanism of action – It involves single electron transfer (SET), which comes from metals and attacks aromatic ring and leads to the formation of the radical anion. The formed anion takes a proton from alcohol to form a neutral radical intermediate. Then again single electron transfer takes place, finally, a proton is abstracted from alcohol to form cyclohexadiene along with metal alkoxide salt byproduct.
If substituted aromatic rings are there then, Birch empirical rules predict regiochemistry.