Beckmann Rearrangement Reaction
It is a rearrangement reaction in which oximes are converted into amides in presence of acid. The reaction was discovered by Ernst Otto Beckmann who was German chemist.
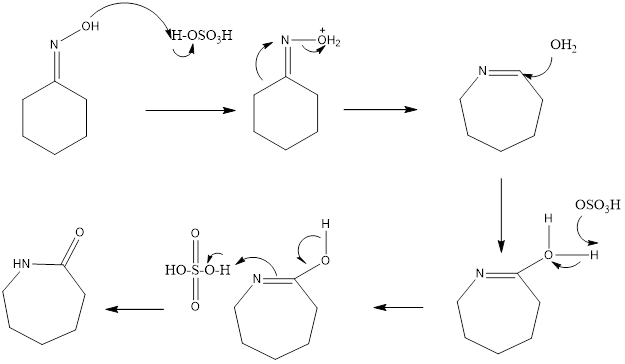
The reaction is acid catalyzed, different acids can be used like polyphosphoric acid, sulfuric acid, hydrogen fluoride, acetic acid, and hydrochloric acid. Mostly sulfuric acid is employed as it forms lactam, which has much commercial importance as it is starting substrate for formation of nylon. It also forms byproduct sulfate which is neutralized by ammonia to form ammonium sulfate which is used as fertilizer.
Mechanism of action– Oxime is formed from ketone then, firstly oxime gets protonated so, that alcoholic group can easily be removed. Then rearrangement step takes place, carbon group trans to leaving group moves to nitrogen. This leads to the formation of carbocation followed by an attack of a water molecule. Then deprotonation occurs followed by tautomerization. At first, nitrogen is protonated, then deprotonation of oxygen occurs to form final amide as a product. This reaction involves the alkyl shift during the rearrangement reaction.
Beckmann rearrangement reaction can also form nitriles if the starting substrate is aldehydes in place of ketone. For formation of nitrile it involves hydride shift as the main step, then nitrogen gets deprotonated to form nitrile.