Baker -Venkataraman Rearrangement
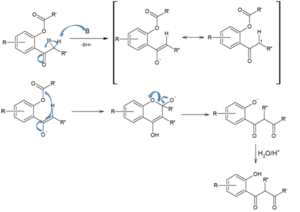
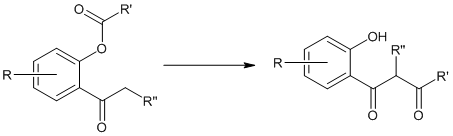
This rearrangement reaction of organic chemistry involves the regio-selective formation of 1,3-diketones through the base-induced transfer of acyl group in O-acylated phenol ester. It is an intramolecular acyl transfer reaction via the formation of an enolate. This rearrangement reaction plays a key role in the synthesis of flavons from readily available starting materials.
Where R’ = Ph, NR2, etc. and R & R’’ = alkyl groups
Though the reaction is regarded as intramolecular claisen condensation, this does not produce any cyclic products.
Mechanism involving in this rearrangement reaction is: