Baeyer-Villiger Oxidation
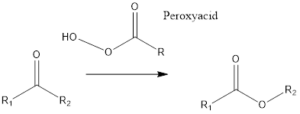
It is the type of organic redox reaction to form ester or lactone from a ketone or cyclic ketone respectively. The reaction takes place in presence of peroxy acid, most commonly 3-chloroperoxybenzoic acid and trifluroperacetic acid are used.
The reaction was first discovered by Adolf von Baeyer and Victor Villiger in the year 1899. They have prepared lactones by using camphor, menthone, and tetrahydrocarvone.
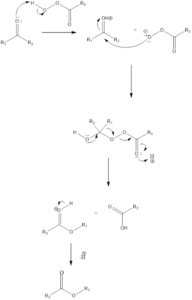
Mechanism of action – Firstly the carbonyl compound is protonated by peroxy acid, which forms a more reactive carbonyl group. The reactive carbonyl group is attacked upon by peroxy acid to form tetrahedral intermediate also known as Criegee intermediate. Then alkyl migration occurs, a group which is more electron rich is migrated towards oxygen of peroxide group and the carboxylic acid is released.
Highly substituted alkyl group attaches to oxygen i.e. 3°alkyl 2° alkyl aryl 1° alkylMethyl. The process predicts product stereochemistry. This step is one of the most crucial steps and it is the rate-determining step. At last, deprotonation occurs of formed oxocarbenium ion to produce the ester.
Baeyer –Villiger reaction is used to synthesize zoapatanol which is used for induction of labor and menstruation and in the synthesis of anti-cancer drug testolactone.