Chemical Properties of Methane (Oxidation)
Methane reacts with reactive substances under vigorous conditions. One such reactive substance is oxygen. Oxidation of methane or in other words combustion (complete) of methane (in fact for any hydrocarbon) results in formation carbon dioxide and water with release of heat. This oxidation is the major reaction occurring during the burning of natural gas.
 Combustion reaction of methane
The heat released when one mole of methane is burned called heat of combustion of methane, which is 213 kcal.
Partial oxidation of methane and catalytic oxidation with water are very important in the synthesis of acetylene, ammonia, methanol and other alcohols.
Combustion reaction of methane
The heat released when one mole of methane is burned called heat of combustion of methane, which is 213 kcal.
Partial oxidation of methane and catalytic oxidation with water are very important in the synthesis of acetylene, ammonia, methanol and other alcohols.
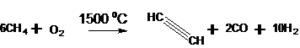 Partial combustion of methane
Partial combustion of methane
 Catalytic oxidation of methane with water
Catalytic oxidation of methane with water
 Combustion reaction of methane
The heat released when one mole of methane is burned called heat of combustion of methane, which is 213 kcal.
Partial oxidation of methane and catalytic oxidation with water are very important in the synthesis of acetylene, ammonia, methanol and other alcohols.
Combustion reaction of methane
The heat released when one mole of methane is burned called heat of combustion of methane, which is 213 kcal.
Partial oxidation of methane and catalytic oxidation with water are very important in the synthesis of acetylene, ammonia, methanol and other alcohols.
 Catalytic oxidation of methane with water
Catalytic oxidation of methane with water 


