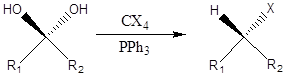
Appel Reaction
It is a type of the organic chemistry reaction to form alkyl halide directly by reaction of tetrahalomethane and triphenylphosphine and alcohol. The reaction was discovered in 1975 by Rolf Appel.
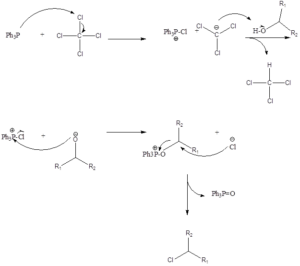
Mechanism – Firstly in reaction triphenylphosphine and tetrachloromethane react to form phosphonium salt. Then deprotonation of alcohol occurs forming alkoxide followed by nucleophilic displacement, where chloride is displaced by alkoxide to form an oxyphosphonium intermediate. Finally, SN2 displacement occurs as halide attacks to form alkyl halide along with by-product triphenylphosphine oxide. Presence of strong P=O is the driving force for the reaction.
It can be used for carboxylic acids which lead to the formation of oxazolines, oxazines, and thiazolines. Appel reaction is used for chlorination of geraniol to geranyl chloride. The reaction is less commonly used as there is the use of toxic halogen agent and by-product triphenylphosphine oxide is organophosphorous product, it requires its separation from the product which is formed.