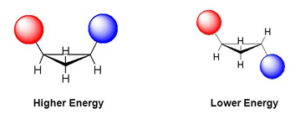
Angle ,Torsional and Steric Strains
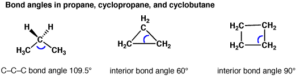
Angle Strain: Any deviation from normal bond angles (for sp3 hybridized carbon it is 109o, for sp2 it is 120 o and for sp hybridized it is 180 o) causes angle strain because the normal bond angle is most stable conformation for a molecule.
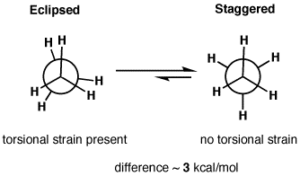
Torsional Strain: Any deviation from staggered conformation causes torsional strain.
Steric Strain: Non-bonded atoms (groups), if brought any closer than normal distance (van der Walls radii) causes repulsion or steric strain.