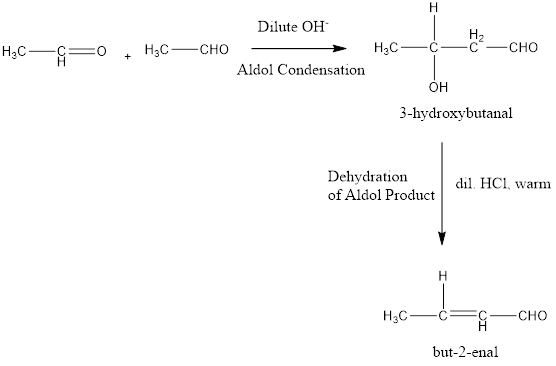
Aldol Condensation
In aldol condensation two molecule of aldehydes or a ketone combine to form a beta hydroxy carbonyl compound under the influence of dilute base. Dilute base abstracts an alpha hydrogen and the generated nucleophile attacks on another molecule of aldehyde or ketone to produce aldol product. These aldol products can be further dehydrated to produce enal or enone compounds, which are stable one due to conjugation of carbon-carbon double bond with carbon-oxygen double bond of carbonyl group.