Absolute Configuration
An arrangement of the atoms in the space attached to a chiral carbon is referred as configuration and most commonly expressed by absolute configuration as “R” and “S.”
The absolute configuration assigned by CIP system given by three organic chemists named as R.S. Cahn, C.K. Ingold, and V. Prelog.
A CIP nomenclature includes the assignment of a prefix R and S to each chiral center in the molecule based on if its configuration is right or left handed respectively.
The symbol R comes from the Latin word “rectus” which means right, and S comes from “sinister” which means left.
The assignment of R/S system is done by the Sequence rule and the viewing rule.
The sequence rule:
The priority is assigned for all four different substituents at asymmetric carbons as follows:
1. Atoms directly attached to the chiral center.
(i) Higher the atomic number of the immediate substituent atom, the higher the priority.
Like: priority order Br- > Cl- > F- > O- > N- > C- > H-
(ii) If there are different isotopes of the same element present that these are assigned a priority according to their atomic mass.
Like: D- > H-
(iii) If two substituents are same, then next atoms are considered to assign the priority base on atomic number
Like: BrCH2– > ClCH2– > C2H5– > CH3
(iv) If double or triple bonded are present in a substituent, they are treated as an equivalent set of single-bonded atoms.
Like: HC≡C- > CH2=CH- > C2H5-
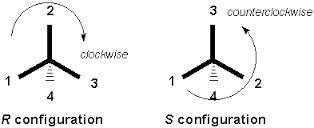
The Viewing Rule
After assigning the relative priorities of the four substituents, the chiral center is viewed opposite side of the lowest priority group (or in other words lowest priority should be away from the viewer). Now top three substituents are in front which is viewed from 1 to 2 and 2 to 3. If viewer rotates eyes clockwise, then it is assigned as “R” or if viewer rotates eyes anticlockwise then it is assigned as “S”.
Example: Lactic acid
It has four different substituents on chiral carbon: H, CH3, OH, and COOH
The relative priorities are 1. OH > 2. COOH > 3. CH3 > 4. H