Gas Chromatography
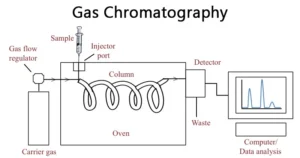
Gas chromatography is also a technique of identifying and isolating a molecule based on its vaporization without any decomposition. Although, the prominent use of this technique is to purify and separating a compound. Moreover, it can be utilized for preparative methods.
The mobile phase in gas Chromatography is usually a gas carrier that is unreactive or inert such as helium and nitrogen. The stationary phase is generally a polymer or microscopic layer over a solid glass. An instrument that is used is called a gas chromatograph.
 History:
As the history of chromatography gave detail about Michael Tswett’s work during 1903. After him, Erika Cremer and Fritz prior in 1947, gave a description of GC and construct the first liquid-gas chromatography. But nobody accepted it and the work remained unrevealed. Then Archer John who was honored with Nobel Prize for paper and liquid-liquid chromatography revealed the GC and its working but it was well known after the discovery of flame ion detector.
Applications:
History:
As the history of chromatography gave detail about Michael Tswett’s work during 1903. After him, Erika Cremer and Fritz prior in 1947, gave a description of GC and construct the first liquid-gas chromatography. But nobody accepted it and the work remained unrevealed. Then Archer John who was honored with Nobel Prize for paper and liquid-liquid chromatography revealed the GC and its working but it was well known after the discovery of flame ion detector.
Applications:
 History:
As the history of chromatography gave detail about Michael Tswett’s work during 1903. After him, Erika Cremer and Fritz prior in 1947, gave a description of GC and construct the first liquid-gas chromatography. But nobody accepted it and the work remained unrevealed. Then Archer John who was honored with Nobel Prize for paper and liquid-liquid chromatography revealed the GC and its working but it was well known after the discovery of flame ion detector.
Applications:
History:
As the history of chromatography gave detail about Michael Tswett’s work during 1903. After him, Erika Cremer and Fritz prior in 1947, gave a description of GC and construct the first liquid-gas chromatography. But nobody accepted it and the work remained unrevealed. Then Archer John who was honored with Nobel Prize for paper and liquid-liquid chromatography revealed the GC and its working but it was well known after the discovery of flame ion detector.
Applications:
- It is used in forensic labs, for the detection of poisons and drug toxicology.
- Pollutant detection in drinking and wastewater.
- Metabolites study in blood and urine samples.
- Identify unknown hazardous material in waste dumps.
- To analyze the reaction product for industries in quality control.
- Only fixed gas analysis, this is why fewer applications.
- The efficiency of the column is less than capillary.
- Less sensitivity towards those molecules having less affinity for electrons.
- Carrier gas needs to be pure.
- The sample needs to be volatile.
- Impure samples can cause trouble.



