Covalent Bond
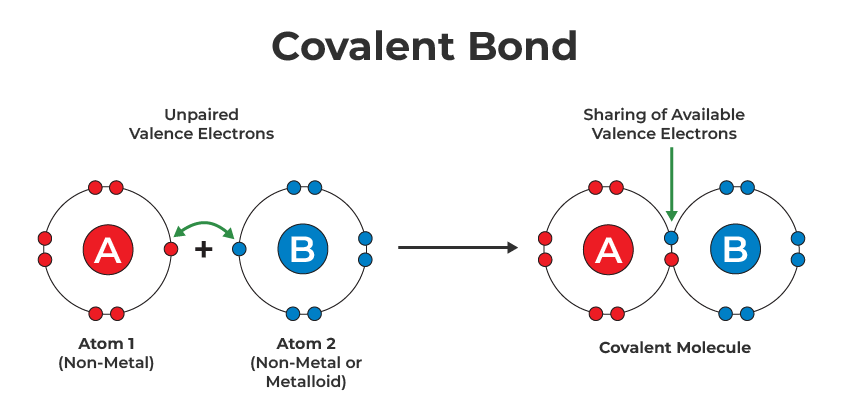
Covalent bonding is a chemical bonding that takes place between atom which shares their electron pair/shared pair/ bonding pairs. It is formed when a stable balance between repulsive and attractive forces takes place. This type of bonding allows each atom participating to attain full configuration.
These types of bonding are very common than that of ionic bonds. It includes many kinds of interactions such as π- bonding, sigma bonding, metal-to-metal bonding, etc. The phenomena are known as co-valency and it is higher in those atoms having the same electronegativity.
Characters of covalent bonds:
- It only formed between 2 non-metals.
- It does not conduct electricity.
- These compounds having low boiling and melting point.
- Compounds are completely soluble in oil and insoluble in water.
- Biological compounds are covalent in nature.