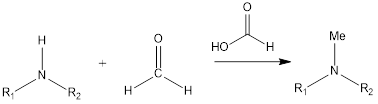
Eschweiler - Clarke Reaction
Eschweiler- Clarke reaction is a substitution organic chemistry reaction which leads to the formation of tertiary methylamines by reaction of primary amine or secondary amine in presence of formaldehyde and formic acid. The reaction was discovered by German chemist Wilhelm Eschweiler as well as British chemist Hans Thacker Clarke.
Mechanism of reaction– Firstly in reaction formaldehyde is protonated, then amine reacts with it to form iminium ion as intermediate. Then the reaction of intermediate and formic acid leads to formation of methylated ammonium ion along with carbon dioxide release, this precedes reaction in forwarding direction. Finally, ammonium ion gets deprotonated to form the product. If primary amine is substrate in reaction then steps are repeated twice to form tertiary product.