Elimination Reactions
As the name indicates, small molecule eliminates during these types of reactions. This small molecule is generally water (-H2O) in the case of dehydration or HX in the case of dehydrohalogenation. A 1, 2-elimination suggests that the atoms that are eliminated
come from adjacent or neighbor carbon atoms. In the organic chemistry, Elimination reactions are the important method for alkenes synthesis.
Following two are the widely described elimination reactions in organic chemistry:
- Dehydration: Elimination of water i.e.-H2O from the Alcohol.
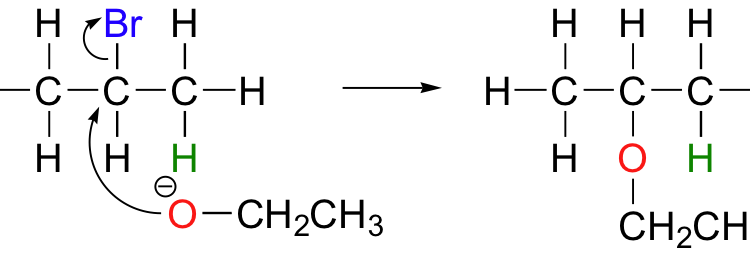
- Dehydrohalogenation: Elimination of HX from alkyl halides. ( where X = Cl, F, Br or I)
- Removal of a hydrogen or proton.
- Formation of the new Carbon-Carbon double bond i.e. pi bond.
- Breaking of Carbon -leaving group bond.
- Removal of leaving the group to form a carbocation, then removal of a proton and formation of the C=C bond. Reactions proceeds via this pathway are referred as E1 reactions.
- Another pathway, simultaneous proton removal and loss of the leaving group to form C=C bond. These types of reactions are referred as E2 reactions.
- Stability of Carbocation.
- Nature of leaving the group.
- Base: Strong or weak base.
| E1 | E2 | |
| Unimolecular/Bimolecular | Unimolecular | Bimolecular |
| Rate depends on | The concentration of Substrate. | The concentration of Substrate and concentration of Base as well. |
| Base | Strong base not needed. | Strong base needed |
| Stereochemistry requirement | Not needed. | Leaving group should be anti to hydrogen that has to remove. |
| Steps | Two step reactions | One step reaction. |
| Carbocation | Should form a stable carbocation. | Not needed. |