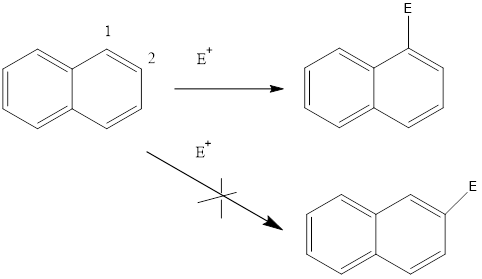
Why Nephthalene Undergoes Electrophilic Aromatic Substitution at Position 1 not 2?


As shown in above figures when electrophile attacks on position 1 it can form two resonating structures while when attack on 2nd position there is no resonating structure possible. Thus, attack on 1st position makes more stable intermediate compared to 2nd position attack.