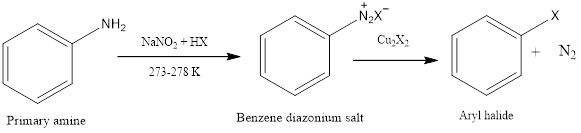
Sandmeyer Reaction
Sandmeyer reaction is a nucleophilic aromatic substitution reaction in which benzene diazonium salt is converted to aryl halide in presence of copper halide. The reaction was first discovered by Traugott Sandmeyer who was a Swiss chemist in the year 1804. Diazonium salt is formed in presence of sodium nitrite and cold aqueous mineral acid. Benzene diazonium salt is used as starting material for formation of aryl halide, then in place of diazonium group, there is the substitution of halide or nucleophile.
Mechanism of action: Diazonium ion is formed by reaction of a primary amine with a nitrous acid. Nitrous acid is prepared by sodium nitrite and acid by protonation. Then with the loss of water, there is the formation of nitrosonium ion. Finally, there is the formation of benzene diazonium salt by electrophilic attack of nitrosonium ion on aromatic amine. Then benzene diazonium salt in presence of copper halide undergoes substitution and the diazo group is replaced by halogen. The mechanism involves the transfer of one-electron and loss of nitrogen gas.
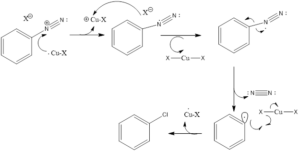
This reaction gives different substitution products involving halogenation, hydroxylation, cyanation, the addition of Iodine group and a sulfhydryl group. Fluorination cannot be done by this reaction, it is performed in presence of tetrafluoroborate anions which is referred to as Balz-Schiemann reaction.