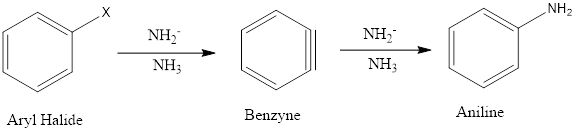
Nucleophilic Aromatic Substitution (SNAr)
Ordinarily aromatic benzene ring and their derivatives give electrophilic aromatic substitution due to the high density of electrons over the ring but nucleophilic aromatic substitution can be seen in some specific conditions like aryl ring must contain strongly electron withdrawing groups on ortho and/or para positions to facilitate the nucleophile attack on the aromatic ring. Only aryl halides undergo Nucleophilic Aromatic Substitution via two possible mechanisms, one is bimolecular displacement, and other is known as an elimination-addition reaction.
Bimolecular displacement:
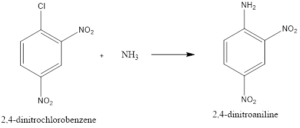 Elimination-addition:
Elimination-addition:
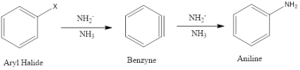
 Elimination-addition:
Elimination-addition: