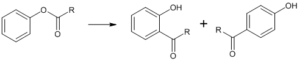
Fries Rearrangement
It is an organic reaction wherein acyl phenols are formed from phenolic esters in the presence of a Lewis acid catalyst.
Many of these are act as intermediates in the pharmaceutical, agricultural, thermographic and other industrial synthetic products.
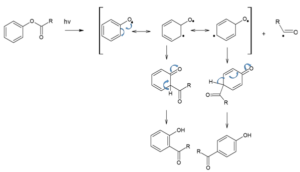
When the same rearrangement reaction takes place in the presence of light, it is known as photo-fries rearrangement.
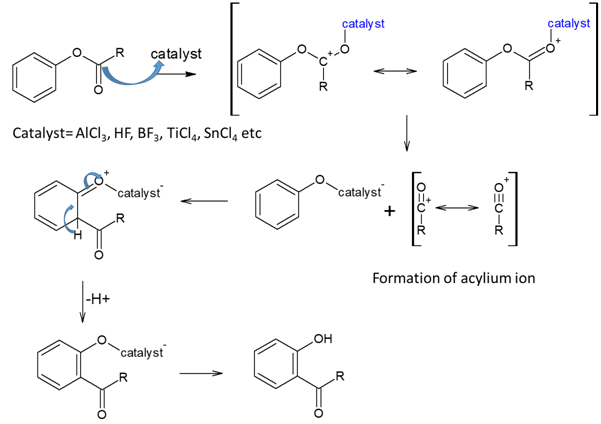
Mechanism of Fries rearrangements is as follows,
When acylium ion attacks p-position, it forms p-product.
Mechanism of Photo-Fries rearrangements is as follows,