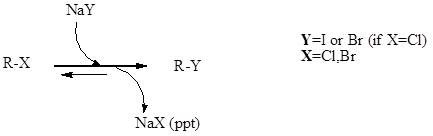Finkelstein Reaction
Finkelstein reaction is a single step substitution nucleophilic bimolecular reaction (SN2) which involves the halide replacement. The reaction was discovered by Hans Finkelstein a German chemist.
It is a type of equilibrium process and it is carried forward as acetone has poor solubility for formed metal halide which involves Le Chatelier’s principle. The reaction is basically used for the formation of alkyl iodide from alkyl chloride or alkyl bromide. In reaction sodium chloride and sodium bromide are not soluble in acetone, but sodium iodide is soluble.
![]()
Mechanism of organic reaction: It is a simple one-step reaction and it involves SN2reaction. There is occurrence of inversion of stereochemistry.
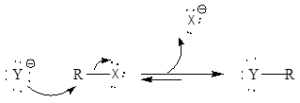
The reaction is dependent upon nature of group whether it is a good leaving group or not, nucleophilicity, alkyl halide reactivity, and carbon-halide bond.
Aromatic Finkelstein Reaction
Aromatic Finkelstein reaction has to be well catalyzed by copper iodide along with diamine ligands as these reactions don’t occur easily. Other catalysts like nickel bromide and n-butyl phosphine can also be used.