Heterocyclic Chemistry
Definition:
Heterocyclic compounds contain nitrogen Sulphur and oxygen in the cyclic ring at the place of carbon. Hence, their common names regard them. They generally have one or more heteroatoms in the ring, and all the nucleic acids are examples of such compounds. Approximately 59 % of the drugs approved by the US FDA contain nitrogen in the ring. Therefore, it is an important field of chemistry, and heterocyclic chemistry homework help professionals are always available for the students.
History:
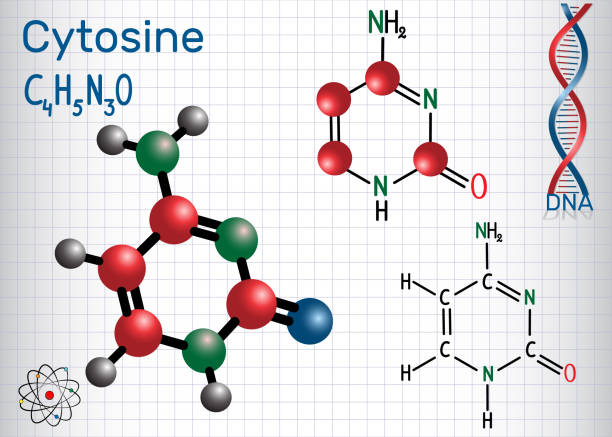
The origin of heterocyclic chemistry was found when organic chemistry developed during 1800. Brugnatelli, in 1818 separated alloxan from uric acid. Later on, in 1832, Dobereiner produced furan compounds using sulphuric acid with starch. Runge collected pyrrole using dry distillation in 1834. Around 1906, Friedlander supersedes the agriculture industry with synthetic chemistry by the production of indigo dye. Tribe discovered petroleum origin by isolating the chlorophyll compounds from natural oil, which took place in 1936. However, this was the earlier origin of heterocyclic chemistry. After that, in 1951, chargaff’s rule was introduced, which shows the use of heterocyclic chemistry in genetic codes such as purine and pyrimidine bases.
Classification:
It can be classified based on the saturation or the saturation of rings and atoms present in the rings. These are discussed below with examples.
- Five-membered ring systems: antimony, arsenic, and bismuth are examples of five-membered rings that contain only one heteroatom. Saturated compounds are stibolane and arsolane, whereas unsaturated are stibole and arsole. A five-membered ring with two heteroatoms is imidazoline (2 nitrogens), Oxathiolidine(oxygen and sulfur), thiazolidine(sulfur and nitrogen), and dioxolane(2 oxygen). These are saturated compounds. It can be studied with heterocyclic chemistry assignment help. Imidazole, oxathiol, and thiazole are unsaturated compounds. Five membered rings with three heteroatoms are triazole, furazan, and oxadiazole unsaturated compounds.
- Six-membered ring system: saturated compounds with one heteroatom are Arsinane and Borinane. Unsaturated compounds with one heteroatom are Arsinin and Bismin. Saturated compounds with two heteroatoms are diazinane and morpholine, while unsaturated compounds with two are diazine and oxazine.
- Seven-membered ring systems: examples of saturated compounds with one heteroatom are azepane and oxepane. Unsaturated compounds with one heteroatom are azepine and oxepine. Two heteroatoms containing saturated molecules are diazepane and unsaturated diazepine.
Importance:
- These are naturally occurring or biosynthesized.
- They are an important part of life, such as chlorophyll for plants and haem.
- Amino acids and nucleic acids are made up of heteroatoms and are essential for living beings.
- Alkaloids like nicotine and caffeine are heterocyclic.
- To treat an antibacterial disease, antibiotics are required. Penicillin and sulfonamides are some of the antibiotics which are made up of heteroatoms.
- To prevent insects, triazole is used, which is a heterocyclic insecticide.
- Triazines and pyridine are herbicides that contain nitrogen in their structure.
- Some hormones and dyes produced in industries include the use of heterocyclic compounds.
- In our food preparations also involves such compounds in the form of carbohydrates, vitamins, and proteins.