A different arrangement of the atoms in the space by rotation about the carbon-carbon single bond is known as conformers and collectively called as conformational isomers. These are inter-convertible by rotation of single bond.
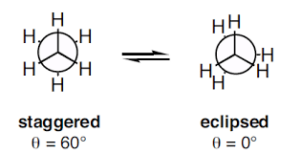
The simplest molecules show conformation is ethane (CH3CH3). The conformer I is called as Staggered conformation and II is called as Eclipsed conformation showed below in Newman projection, whereas intermediate structures are called as Skew conformations.
The potential energy of these conformations reveals that staggered conformation is more stable than Eclipsed conformation by 3 Kcal/mol (due to steric or Van der Wall repulsion between overlapping hydrogen).