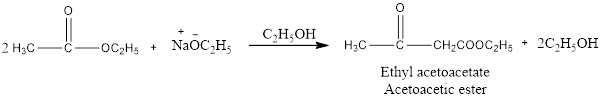
Claisen Condensation
The Claisen Condensation reaction is somewhat different from aldol condensation because it’s a type of substitution reaction while aldol condensation is an addition type of organic reaction. In Claisen condensation two ester molecules condense to form a new C-C bond with the help of a base which abstracts alpha hydrogen from one of the ester molecules and convert it into enolate ion, which attacks on second ester molecule and form a beta-keto ester (Acetoacetic ester) as shown in above figure. If this same reaction takes place in intramolecular form then its known as Dieckmann Condensation. If two different esters is considered for this reaction, then there would be a mixture of four products that would be of less synthetic utility.