Cannizzaro reaction is a type of organic redox reaction, in which two molecules of aldehydes in presence of base converts to their primary alcohol and carboxylic acid by reduction as well oxidation respectively. In this reaction aldehydes should not have any alpha hydrogen (like formaldehyde, benzaldehyde etc.)
The reaction was first discovered in 1853 by Stanislao Cannizzaro, he found at that time formation of benzyl alcohol and potassium benzoate when benzaldehyde was reacted with potassium carbonate.
Mechanism of action: Firstly hydroxide attack on carbonyl group, gives intermediate 1 and then addition of hydride ion from this intermediate 1 to a second molecule of aldehyde results in the formation of carboxylate anion and alkoxide. Alkoxide takes a proton from water to form alcohol and carboxylate anion forms sodium carboxylate salt which can be converted to carboxylic acid by acid work up.
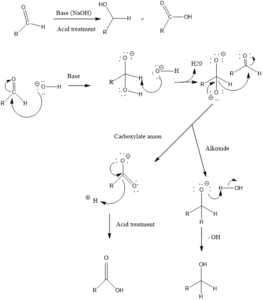
One mole of aldehyde gives 50% of each acid and alcohol as a product.