Baylis - Hillman Reaction
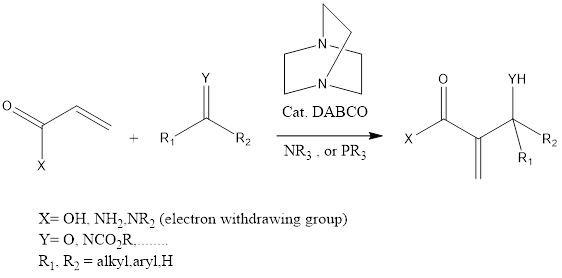
Baylis-Hillman reaction is a coupling organic chemistry reaction that leads to forming a C-C bond in between α, β carbonyl compounds like aldehyde or activated ketone and electrophiles. The reaction takes place in the presence of tertiary amine and phosphine and mostly DABCO (1,4 Diazabicyclo [2.2.2] octane tertiary amine is used.
The reaction has been discovered by Anthony B. Baylis and Melville E.D Hillman in 1972. The reaction is also referred to as Morita-Baylis-Hillman reaction or MBH reaction.
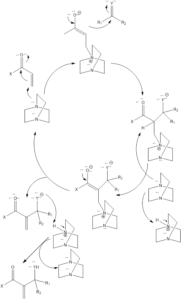
Mechanism of reaction– Firstly carbonyl compound gets activated by a catalyst which leads to the formation of nucleophilic anion, which further attacks electrophile. The reaction is proceeded ahead to form the desired product. The catalyst gets regenerated in the reaction which undergoes again cycle of catalysis.