Bartoli Indole Synthesis
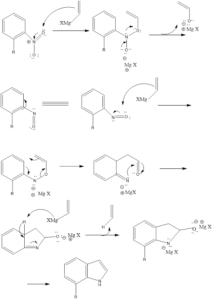
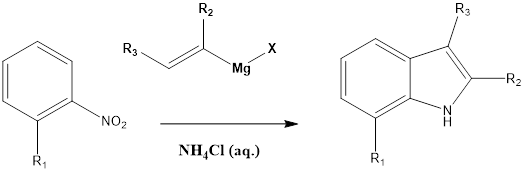
This reaction involves the formation of substituted indoles from nitroarene in presence of an excess of vinyl Grignard reagent and aqueous ammonium chloride. The reaction was discovered by Giuseppe Bartoli and his team in the year 1989.
The ortho-substituted nitroarene is preferred as they give a good yield. The bulkier group also affects yield, as it leads to higher yields of reaction. The bulkier ortho group helps in 3,3 sigmatropic rearrangement, which forms the final product. For the formation, three equivalents Grignard reagent is required.
Mechanism of reaction: The reaction begins with the attack of Grignard reagent on nitroarene then 3,3 sigmatropic rearrangement occurs leading to the formation of aldehyde intermediate. Then nitrogen attacks aldehyde readily, intramolecularly and then the third equivalent of Grignard reagent attacks to form the aromatic ring and finally there is the formation of the product via acid workup.