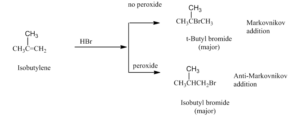
Anti - Markovnikov Rule
The addition of hydrogen halide to an alkene in the presence of peroxide is opposite to Markovnikov rule.
This reversal of orientation of product is peroxide effect and also known as Kharasch effect observed by M.S. Kharasch and F.R. Mayo in 1973.
So, in the presence of peroxide addition of hydrogen on carbon-carbon double bond that holds less number of hydrogen.
Examples:
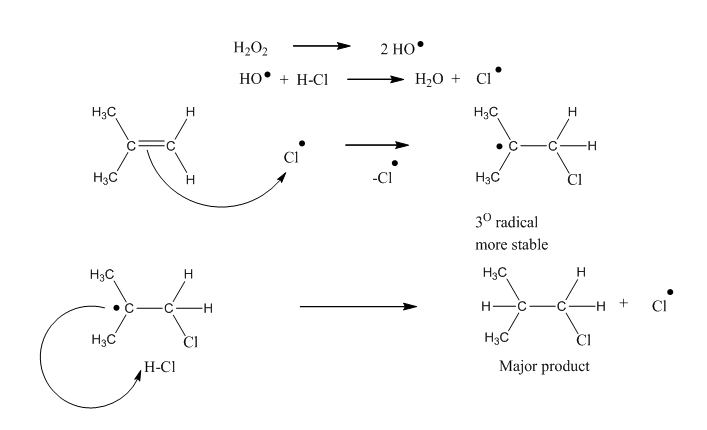
 Mechanism:
It’s a free radical addition reaction and involves the formation of free radical. The more stable free radical (3° > 2° > 1°) defines the orientation of halogen.
Mechanism:
It’s a free radical addition reaction and involves the formation of free radical. The more stable free radical (3° > 2° > 1°) defines the orientation of halogen.
 Mechanism:
It’s a free radical addition reaction and involves the formation of free radical. The more stable free radical (3° > 2° > 1°) defines the orientation of halogen.
Mechanism:
It’s a free radical addition reaction and involves the formation of free radical. The more stable free radical (3° > 2° > 1°) defines the orientation of halogen.