Mannich Reaction
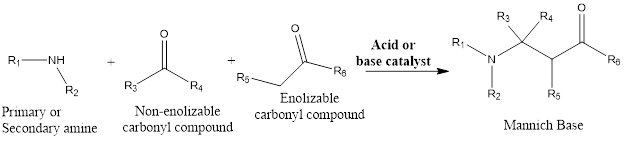
The mannich reaction is an organic amino alkylation reaction which leads to the formation of a β-aminocarbonyl compound from primary or secondary amine and carbonyl compounds from which one should be enolizable and other the other one should be non-enolizable. The reaction takes place in presence of base or acid catalyst. The reaction was discovered by Carl Mannich, that’s why the reaction is named as a Mannich reaction.
Mainly reaction involves primary or secondary amine, formaldehyde as non-enolizable carbonyl compound and the alpha hydrogen containing enolizable carbonyl compound.
Mechanism of action: Carbonyl compounds get protonated in acid catalyst reaction at oxygen site. The enolizable compound having α-hydrogen is deprotonated leading to the formation of enol intermediate. Formaldehyde or non-enolizable compound with amines form iminium ion. The iminium ion is attacked by enol intermediate, then deprotonation occurs which finally leads to the formation of β-amino carbonyl compound which is also known as the Mannich base.
The Mannich reaction is involved in many biosynthetic pathways like in alkaloid formation, in many organic synthesis reactions for formation of polymers, catalyst, alkyl amines, drugs like tramadol and fluoxetine etc. This reaction is also used in the epoxy coating and in the formation of soaps/detergents.