Schmidt Reaction
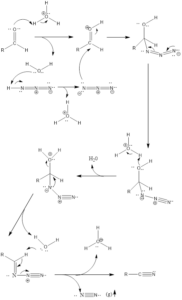
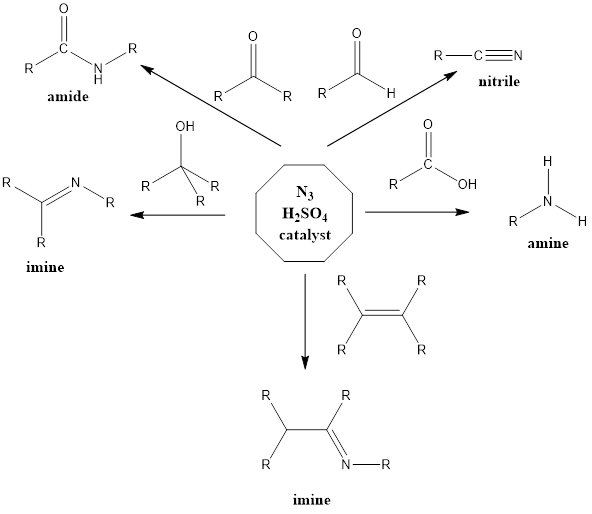
It is a rearrangement organic reaction where electrophiles like carbonyls, tertiary alcohols or alkenes react with hydrogen azide to form various products like amines, nitriles, amides or imines with the expulsion of nitrogen. The reaction was discovered by Karl Friedrich Schmidt in 1924 where benzanilide was successfully formed from benzophenone and hydrazoic acid.
Reaction Mechanism of nitrile formation – The mechanism of action is flexible extends to all reagents. With aldehyde reagent nitrile is formed and reaction firstly involves abstraction of a proton from the acid by aldehyde or by any other reagent. From water molecule formed, proton gets abstracted from hydrazoic acid which leads to the formation of azide anion, further activated reagent is attacked upon by azide anion. Finally, there is the formation of a final product through a series of steps along with the release of nitrogen gas.