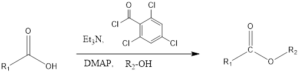
The Yamaguchi esterification is the coupling organic chemistry reaction where the ester is formed from a carboxylic acid in presence of alcohol, triethylamine, 2,4-trichlorobenzoyl chloride i.e. Yamaguchi reagent and DMAP (4-Dimethylaminopyridine). The reaction was discovered in 1979 by Masaru Yamaguchi.
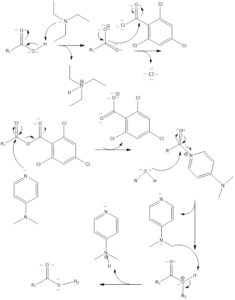
Mechanism of action– Firstly carboxylic acid get deprotonated by triethylamine which results into formation of carboxylate anion. The carboxylate anion formed attacks 2,4-trichlorobenzoyl chloride and this results to form acid anhydride which is further attacked upon by 4- dimethylaminopyridine. The 4-Dimethylaminopyridine is then replaced by alcohol. Lastly, deprotonation occurs, which results in the formation of an ester.